NANO-THERMAL BIOENGINEERING LABORATORY
RESEARCH AREAS
MOLECULAR HYPERTHERMIA
Precise manipulation of protein activity in living systems has broad applications in biomedical sciences. However, it is challenging to use light to manipulate protein activity in living systems without genetic modification. Molecular hyperthermia (MH) uses the nanoscale plasmonic heating of gold nanoparticles (AuNPs) to thermally inactivate targeted proteins. We showed that MH can inactivate PAR2, a G-protein-coupled receptor implicated in pain, and JAM-A, one of the tight junction proteins in the blood−brain barrier. As a protein inactivation method that does not rely on genetic modifications, MH will find many applications in biomedical sciences.
Support: NIH
Relevant Publications:
1. Kang, P., Chen, Z., Nielsen, S.O., Hoyt, K., D’Arcy, S., Gassensmith, J. and Qin Z. Molecular Hyperthermia: Spatiotemporal Protein Unfolding and Inactivation by Nanosecond Plasmonic Heating. Small. 1700841. (2017)
2. Daipayan Sarkar, Peiyuan Kang, Steven O. Nielsen, Zhenpeng Qin* Non-Arrhenius Reaction-Diffusion Kinetics for Protein Inactivation over a Large Temperature Range, ACS Nano. (2019)
3. Peiyuan Kang, Xiaoqing Li, Yaning Liu, Stephanie I. Shiers, Hejian Xiong, Monica Giannotta, Elisabetta Dejana, Theodore John Price, Jaona Randrianalisoa, Steven O. Nielsen, and Zhenpeng Qin* Transient Photoinactivation of Cell Membrane Protein Activity without Genetic Modification by Molecular Hyperthermia, ACS Nano. (2019)
4. Peiyuan Kang,# Chen Xie,# Oumar Fall, Jaona Randrianalisoa, Zhenpeng Qin*
Computational investigation of protein photoinactivation by molecular hyperthermia, Journal of Biomechanical Engineering. (2021)
MICRO/NANOSCALE INTERFACE WITH THE BRAIN
UNCAGING TECHNIQUES FOR NEUROMODULATION
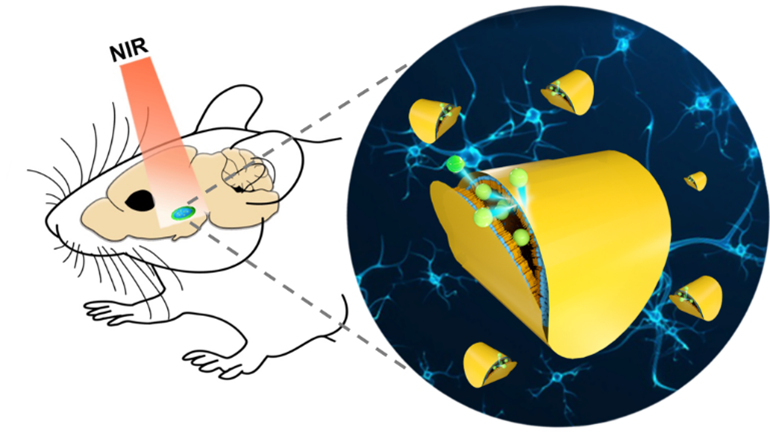
Understanding the signal transmission and processing within the central nervous system (CNS) is a grand challenge in neuroscience. Engineering macro/nanomaterial transducers-based tools with high spatiotemporal control and deep brain penetration are of high interest to us. We have developed near-infrared light responsive plasmonic liposomes to release neuromodulators in an ultrafast speed (<0.1 ms) to study fast cell signaling processes. By adapting stress sensitive vesicles, we were able to extend this technique to uncage neuromodulators in deep brain regions. The ongoing work of this area includes 1) understanding the fate of neuromodulators in the brain circuits, e.g., neuropeptide diffusion; 2) studying the role of neuromodulators on brain circuits and animal behavior; 3) investigating the effects of neuromodulators on some CNS disease, e.g, brain glioma.
Support: NSF
Relevant Publications:
1. Li, X., Che, Z., Price, T. and Qin Z. Ultrafast Near-Infrared Light-triggered Intracellular Uncaging to Probe Cell Signaling. Advanced Functional Materials, (2016)
2. Randrianalisoa, J., Li, X., Serre, M. and Qin Z., Understanding the Collective Optical Properties of Complex Plasmonic Vesicles. Advanced Optical Materials, (2017)
3. Xiuying Li, Peiyuan Kang, Zhuo Chen, Sneha Lal, Li Zhang, Jeremiah J. Gassensmithbd and Zhenpeng Qin* Rock the nucleus: significantly enhanced nuclear membrane permeability and gene transfection by plasmonic nanobubble induced nanomechanical transduction, Chemical Communications. (2018)
4. Karim, M.R., Li, X., Kang, P., Randrianalisoa, J., Qin, Z.* and Qian, D.*, Ultrafast Pulsed Laser Induced Nanocrystal Transformation in Colloidal Plasmonic Vesicles, Advanced Optical Materials, (2018)
5. Hejian Xiong, Xiuying Li, Peiyuan Kang, John Perish, Frederik Neuhaus, Jonathan Ploski, Sven Kroener, Maria O. Ogunyankin, Jeong Eun Shin, Joseph A. Zasadzinski, Hui Wang, Paul Slesinger, Andreas Zumbuehl, Zhenpeng Qin*
Near-infrared Light Triggered-release in Deep Brain Regions Using Ultra-photosensitive Nanovesicles, Angewandte Chemie International Edition. (2020)
BLOOD-BRAIN BARRIER MODULATION
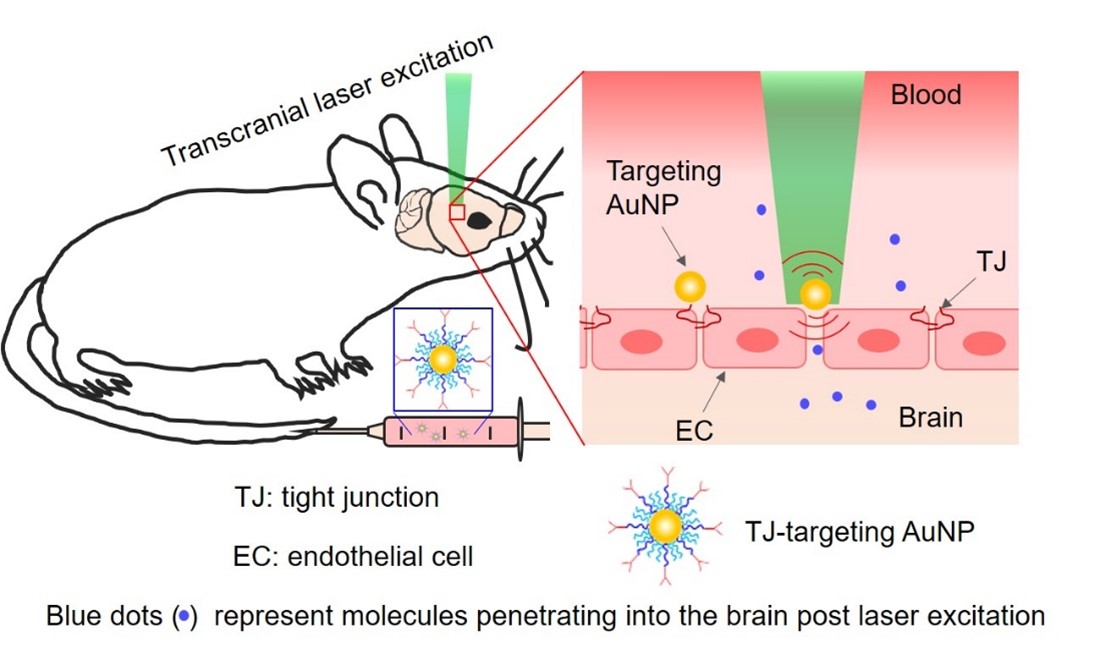
The blood–brain barrier (BBB) poses a major obstacle for drug delivery to the central nervous system. Research in the BBB area focuses on developing novel and safe methods to modulate the BBB for therapeutics delivery. We have developed a simple method to temporarily increase the BBB permeability by using picosecond laser stimulation of tight junction-targeted gold nanoparticles (AuNPs) without disruption of the structure or function of the neurovascular unit. We showed the feasibility of using this approach in the delivery of immunoglobulins (IgG), viral gene therapy vectors, AAV, and drug-carriers nanoparticle, liposome.
Support: CPRIT
Relevant Publications:
1. Xiaoqing Li,# Vamsidhara Vemireddy,# Qi Cai,# Hejian Xiong, Peiyuan Kang, Xiuying Li, Monica Giannotta, Heather Hayenga, Edward Pan, Shashank Sirsi, Celine Mateo, David Kleinfeld, Chris Greene, Matthew Campbell, Elisabetta Dejana, Robert Bachoo,* Zhenpeng Qin*
Reversibly Modulating the Blood–Brain Barrier by Laser Stimulation of Molecular-Targeted Nanoparticles, Nano Letters. (2021)
POINT-OF-CARE DIAGNOSIS FOR INFECTIOUS DISEASES
DIAMOND
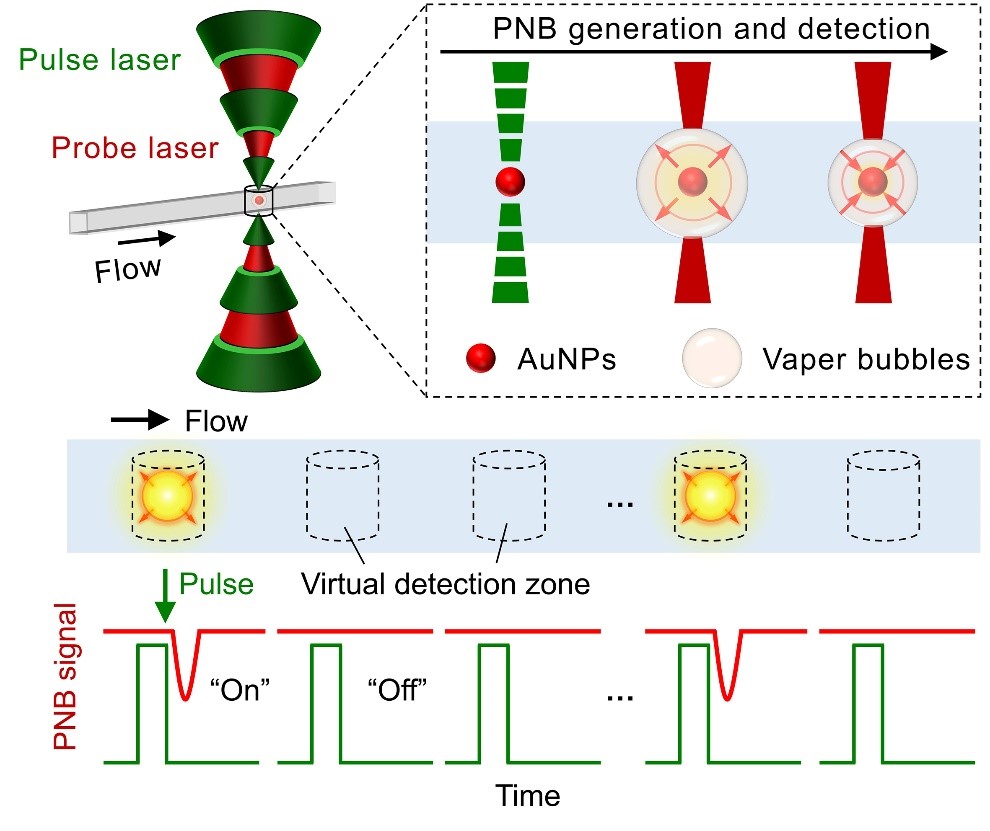
The ultimate goal in diagnostics is to achieve “single-molecule” detection with rapid readout. Towards this, our DIgitAl plasMONic nanobubble Detection (DIAMOND) technique allows single particle detection by analysis of transient nanobubble events created by plasmonic gold nanoparticles-laser interaction in a compartment-free digital counting manner. The ongoing work of this area includes assay innovation, high-throughput and multiplexing detection, as well as clinical applications.
Support: NIH
Relevant Publications:
1. Godakhindi, V.S. *, Kang, P. *, Serre, M. *, Revuru, N.A., Roner, M., Kahn, J., Randrianalisoa, J. and Qin Z. Tuning the gold nanoparticle colorimetric assay by nanoparticle size, concentration, and size combinations for oligonucleotide detection. ACS Sensors. 2017 (*co-first authors)
2. Haihang Ye, Yaning Liu, Li Zhan, Yilin Liu, Zhenpeng Qin* Signal amplification and quantification on lateral flow assays by laser excitation of plasmonic nanomaterials, Theranostics. (2020)
3. Yilin Liu, Li Zhan, Zhenpeng Qin, James Sackrison, and John C. Bischof*
Ultrasensitive and Highly Specific Lateral Flow Assays for Point-of-Care Diagnosis, ACS Nano. (2021)
4. Yaning Liu,# Haihang Ye,# HoangDinh Huynh, Peiyuan Kang, Chen Xie, Jeffrey S. Kahn, Zhenpeng Qin*
Single-Particle Counting Based on Digital Plasmonic Nanobubble Detection for Rapid and Ultrasensitive Diagnostics, medRxiv. (2021)
LAB PHOTO GALLERY
Click on any gallery image to view in full.
WET LAB
The wet lab is equipped with instruments necessary for nanoparticle synthesis, functionalization, and characterization. Major instruments include:
- Beckman DU 800 UV/Vis Spectrophotometer, wavelength range 190 to 1100nm, for protein quantification and nanoparticle spectrum measurement.
- Malvern Zetasizer Nano S90 dynamic light scattering system.
- Biotek Synergy 2 plate reader for absorbance and fluorescence.
- Millipore Direct-Q3 UV ultrapure water system.
- One chemical fume hood.
- Eppendorf 5415D micro centrifuge.
- Fisher Scientific Vortex.
- Precision Model 280 Water Bath.
- Two analytical balances (Mettler Toledo XSE205DU and Adams Nimbus 254e).
- One large glass door laboratory refrigerator (4 ºC).
- One undercounter freezer (-20ºC).
- -80 Freezer.
- Branson digital heated ultrasonic bath.
- Digital temperature hot plate.
- Micropipette puller (Sutter, P-1000).
GENERAL WORKING AREA
REPRESENTATIVE EQUIPMENT IMAGES
BIO-LAB
The cell and tissue culture space consists of one 6-ft biosafety cabinet, two CO2 incubators, and supporting instruments (centrifuge tec.).
Animal preparation: The animal preparation space consists of stereotactic, isoflurane system with oxygen concentrator, nanoinjection robot, and surgical platform. Major instruments include:
- Model 940 Small Animal Stereotaxic Instrument (KOPE)
- DC temperature controller
- OSADA EXL-M40 drilling system
- ZEISS Stemi 508 Greenough Stereo Microscope
- Pureline® M6000 Rebreathing Mobile Anesthesia Machine (Supera)
- Leica VT1200 Semi-Automatic Vibrating Blade Microtome
- 980 nm Diode Laser System
- Neurophotometrics system (Fiber Photometry)
GENERAL WORKING AREA
REPRESENTATIVE EQUIPMENT FIGURES
OPTICS LAB
The optics lab is equipped with lasers, optics, and microscope. Major instruments include:
- A Newport optical table with vibration isolators (model RS400-510-12).
- A high-power mode-locked solid-state picosecond (28 ps) laser with tunable wavelength including 532nm and 680nm--2300nm (Altos Photonics PL2231-100-SH and PG501A).
- A high-power nanosecond laser (Quantel Q-smart 450) and Opotek MagicPRISM optical parametric oscillator (OPO).
- Laser and optics related accessories including energy meter and spectrometer.
- Olympus Inverted and upright microscopes (IX73 and BX51WI) for bright field, fluorescent, polarization and phase and DIC imaging.
- One complete in-vitro electrophysiology set-up integrated with the upright microscope, including amplifier (Multiclamp 700B), manipulators (Sutter, Novato, CA), stimulators etc.
- Primary user of the multiphoton intravital microscopy at Imaging core (with two 2p lasers for simultaneous stimulation and imaging).
- Mightex mesoscope.
LASER SYSTEM
UTD IMAGING AND HISTOLOGY CORE
-
Leica ASP300 S: Fully enclosed Tissue Processor.
-
Leica EM UC7: Ultramicrotome used for sample preparation for electron microscopy.
-
Leica RM2235: Manual microtome.
-
Leica CM1860: Clinical Cryostat.
-
Leica EM FC7: Cryochamber used for sample preparation for electron microscopy.
-
HistoCore ARCADIA C and H: Cold and hot paraffin embedding stations.
-
Leica EM ACE200: Carbon coater used for sample preparation for electron microscopy.
-
Leica EM KMR3 Glass Knifemaker used for sample preparation for electron microscopy.
-
NuAire Class II, Type A2 Biological Safety Cabinet.
-
NuAire Direct Heat In-Vitrocell Air-Jacketed CO2 Incubators.
-
Lab refrigerators and Freezers.
UTD OLYMPUS AND ZEISS IMAGING CENTER
-
Olympus MPE-RS TWIN: Intravital Multiphoton Fluorescence Microscope with dual multiphoton laser system for simultaneous scanning and excitation.
-
Olympus FV3000RS: Laser Scanning Confocal Microscope equipped with live cell imaging capacity and a fully automated stage for whole tissue stitching. The system also includes objectives for cleared tissue imaging compatible with SCA\E and Clarity techniques.
-
Olympus SD-OSR: Spinning Disk Confocal Microscope equipped with live cell imaging capacity.
-
Olympus IX83 TIRF: Total Internal Reflectance Fluorescence system.
-
Olympus VS120: 100-Slide Scanning System.
-
Imaris software work-station for visualizing and analyzing imaged data.
-
Zeiss E-SEM: Environmental Scanning Electron Microscope.